
Properties & composition of stainless steel as shipbuilding materials for chemical tankers
Iron, steel Mild steel and high tensile steel is, and will continue to be the most important material in the building of chemical
tankers and their cargo tanks.Steel is attacked by only a few products, mainly acids and, of course ballast and washing water.
Steel itself contaminates very few products, one of them being high purity caustic soda.
Rust, however, creates many problems with cargo contamination. Rust may contaminate a product in the form of minute particles staying suspended in viscous heavy liquids such as glycols and caustic soda. Rust may accelerate polymerization in polymerizable products.
Rust will be soaked in with remains of previous cargoes, mainly from heavy oils, which will contaminate the next cargo. Rust will, above all, render tank cleaning much more difficult, cause delays and perhaps cargo claims.
Rust, however, creates many problems with cargo contamination. Rust may contaminate a product in the form of minute particles staying suspended in viscous heavy liquids such as glycols and caustic soda. Rust may accelerate polymerization in polymerizable products.
Rust will be soaked in with remains of previous cargoes, mainly from heavy oils, which will contaminate the next cargo. Rust will, above all, render tank cleaning much more difficult, cause delays and perhaps cargo claims.
Due to the above reasons steel is practically never used in chemical tankers without a protective coating .
Coating techniques have advanced greatly in latter years. Still, however, one does not dare to use coatings for products,
which are really aggressive to steel, e g. acids (such as phosphoric acid). One has to reckon with coating defects where corrosion will start.
One exception to this is rubber linings, which have been in use a long time with corrosive cargoes.
Generally speaking, steel is resistant to alkalies, even in high concentrations (caustic soda, ammonia).
Use of stainless steel
Stainless steel has increased greatly in use as tank material in recent years. The motif is not only a better chemical resistance but primarily it provides for a greater ease in tank cleaning and inspection. Thus cargo contamination hazards can be reduced.
The stainless properties are due to the formation of a very thin, passive layer of chromium oxide on the surface. The care of stainless steel tanks aims at maintaining this protective film intact.
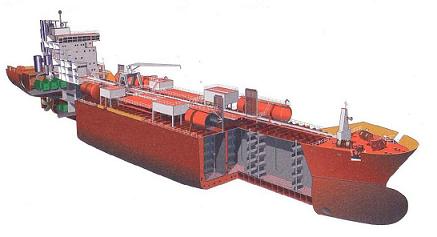
Fig: Typical modern chemical tanker cross section
Stainless steels possess a number of advantageous properties, rustproofness, however, is not guaranteed. It depends on the
correct treatment of the tanks and on what products carried. A short review may be of interest.
The commonly used stainless steels have the following typical compositions:
Low carbon contents are required to make the steel corrosion resistant near the welds. Carbon tends to bind chromium to form chromium carbide in the heat affected zone near the welds, thus reducing the corrosion resistance locally. The addition of titanium has a neutralizing effect on this process and therefore slightly higher carbon contents can be tolerated. The addition of small amounts of nitrogen increases the tensile strength of the steel considerably. Such steels are often used in more highly stressed parts such as corrugated bulkheads subjected to corrosion from both sides, where weight reduction is at a premium.
General corrosion of stainless steels from corrosive cargoes such as phosphoric acid is of a low intensity and can therefore be disregarded.
Pitting corrosion might cause severe damage. To avoid this happening it is important to choose the correct type of stainless steel. Generally speaking high molybdenium contents is beneficial in this respect. Pittings may take the shape of small holes, 1-2 mm in depth and diameter to begin with. At times they are almost hidden below the surface of the steel with an "entrance hole" of only a few tenths of a mm. with a cavity of 1-2 mm below. They are therefore sometimes very difficult to detect. So called dye-penetrant tests are useful for detection when one knows approximately where to look for defects. Pitting may develop in a generally corrosive surrounding, especially when the surface is disturbed or when some extra chemical aggressive age.nt is present such as:
i) chlorides (seawater, "salt"). Contents above some 100-200 ppm are generally dangerous when together with some other corrosive agent such as phosphoric acid. Fluorides have a similar effect.
ii) particles of iron-or other materials on the surface
iii) craters or pores in weld deposits
iv) weld slag or slag from rolling mill
v) surface defects, micro cracks, rough surfaces vi) lack of oxygen renders formation of chromium oxide difficult
vii) high temperatures (above ca 400C corrosion rates in crease rapidly)
Crevice corrosion may occur in narrow spaces where the corrosive agent can enter but without circulation, with a lack of oxygen as consequence.
Typical locations:
a) under bolted connections
b) under cargo sediments
c) under paint on a stainless steel surface.
Maintenance of stainless steel
The following rules for maintenance of stainless steel apply:
1. Keep chlorides away. Avoid seawater in the tanks and rinse with freshwater carefully after seawater washing. Seawater must never be permitted to dry up and leave salt crusts on the surface. Keep the hatch covers closed and the airpipes protected to prevent seawater or a saline atmosphere entering the tanks. Preferably change footwear before entering a tank when at sea.
2. Remove any particles or sediments such as rust, particles from grinding operations, cargo sediments (phosphoric acid), "scale". Cargo remains to be removed as soon as possible after discharge.
3. Surface finish. Tank surfaces to be kept bright and free from scratches. This means-that possible corrosion or other mechanical defects should be ground and polished to the original finish. Normally grinding disc "grain 80" can be used, followed by a final operation with "grain 120". Local pittings of substantial depth can be welded, minor pittings ground away.
4. Inspect for corrosion after each cargo, especially tank bottoms and under deck. In order to avoid salt crystals forming on the tank bottom 10-20 cm fresh water is sometimes kept in the tanks on the ballast voyage.
5. Cleaning can normally be carried out with all common cleaning agent's such as "emulsifiers", "solvent cleaners" and alkaline cleaners as well as caustic soda.
6. Stainless steel tanks are sometimes passivated by application of 12- 15 % nitric acid (HNO 3) . This acid is strongly oxidizing. The procedure assists in building up the passive chromium layer on the steel, thus increasing its chemical resistance. Passivation is normally carried out after tank surface repairs in order to assist the normal passivation in air. If aggressive cargoes are to be loaded within 24 hours all repairs must be passivated. In practice "passivation" with nitric acid is often used for the removal of discolorations and particle contaminations on the surface. In factlit is being more used as a thorough cleaning agent than a passivating chemical. Passivation is normally carried out with a brush or, for a whole tank, by spraying, After 10-20 min the acid should be washed off with large amounts of fresh water.
IMPORTANT: Nitric acid gives off nitrous gases which are very toxic (with delayed effects). Ventilate completely and use breathing masks for larger areas in confined spaces! The atmosphere can be tested for nitrous gases by means of test tubes . Use protective clothing and goggles. Nitric acid is usually available in 60 1/o concentration. Be careful: spills may cause self-ignition of organic matter.
7. Pickling is the toughest way of cleaning stainless steel. This method is used for the removal of welding slag, oxides and discolorations from welding or discoloration from cargoes. Pickling paste, consisting of, among other components, nitric acid and hydrofluoric acid shall be applied with the same precautions as nitric acid above. Pickling should be followed by passivation with nitric acid. Pickling involves a lot of work and can only be used on relatively small areas, unless carried out by specialists.
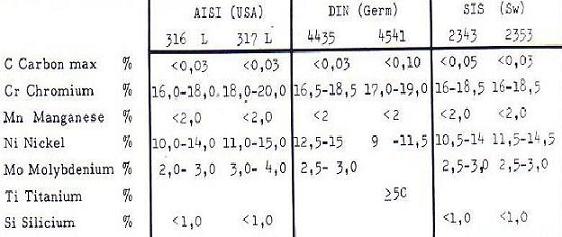
Fig : Steel composition
Other Info pages
Cuprous alloys for shipbuilding of seagoing chemical tankers
Copper and its alloys corrode in many cargoes and may contaminate them, e g styrene, phenol, vinyl chloride, aniline, ammonia solutions etc. Check your cargo against information . Particularly aggressive are the ammonia compounds; they cause inter -crystalline corrosion of cuprous alloys very rapidly. The object in question disintergrates very soon. ....
Use of magnesium and alluminium alloys as sacrificial anodes
Alloys of magnesium and aluminium should never be used in the cargo tank area, due to their poor corrosion resistance in such environments. .....
Introducing rubber lined tanks for the transport of phosphoric acid, waste acids and hydrochloric acid
In recent years a number of ships have been fitted with rubber lined tanks, for the transport of phosphoric acid, waste acids and hydrochloric acid. .....
Tools for shipboard maintenance
Although grit blasting and the use of mechanically powered tools are not normally considered to fall within the definition of hot work, both these operations should only be permitted under controlled conditions. ......
Sea transport of various liquid chemicals at sea
Chemical tankers primarily transport organic and inorganic chemicals as well as vegetable oils and fats. The total global volume of chemicals is estimated at approximately 60 million metric tonnes per year. In addition, the transportation of vegetable oils, alcohols, molasses and lubricating oils amounts to 40 - 45 million tonnes per year. .....
stainless steel as shipbuilding materials for chemical tankers
Iron, steel Mild steel and high tensile steel is, and will continue to be the most important material in the building of chemical tankers and their cargo tanks.Steel is attacked by only a few products, mainly acids and, of course ballast and washing water. Steel itself contaminates very few products, one of them being high purity caustic soda. .... ....
Types of various chemical tankers at sea
modem chemical tanker is primarily designed to carry some of the several hundred hazardous products now covered by the IMO Bulk Chemical Codes. The following general types of chemical carriers have developed since the trade began: ....
Main Info pages!
Home page ||| Chemical hazards ||| Cargo planning & Stowage ||| Cargo loading ||| Cargo documents ||| Safe stability ||| Cargo care ||| Preparation for unloading ||| Inert gas systems |||Gas freeing ||| Nitrogen handling ||| Chemical handling Safe practice |||Handling equipments ||| Cargo & Ballast pumps ||| Cargo tanks |||Tank cleaning |||Special cargoes |||Spills emergencies |||Fire protection

Chemicaltankerguide.com is merely an informational site about various aspects of chemical tankers and safety tips that may be particular value to those working in: Chemical Handling, Chemical Storage, Liquefied Chemical Suppliers, Chemical Shipping, Chemical Transportation, Chemical Terminals, Bulk Chemical Services and Chemical Processing. If you are interested in finding out more about chemical tanker guideline please visit IMO official website. For any comment please Contact us
Copyright © 2011 Chemical Tanker Guide.com All rights reserved.

Use of stainless steel
Stainless steel has increased greatly in use as tank material in recent years. The motif is not only a better chemical resistance but primarily it provides for a greater ease in tank cleaning and inspection. Thus cargo contamination hazards can be reduced.
The stainless properties are due to the formation of a very thin, passive layer of chromium oxide on the surface. The care of stainless steel tanks aims at maintaining this protective film intact.
Fig: Typical modern chemical tanker cross section
The commonly used stainless steels have the following typical compositions:
Low carbon contents are required to make the steel corrosion resistant near the welds. Carbon tends to bind chromium to form chromium carbide in the heat affected zone near the welds, thus reducing the corrosion resistance locally. The addition of titanium has a neutralizing effect on this process and therefore slightly higher carbon contents can be tolerated. The addition of small amounts of nitrogen increases the tensile strength of the steel considerably. Such steels are often used in more highly stressed parts such as corrugated bulkheads subjected to corrosion from both sides, where weight reduction is at a premium.
General corrosion of stainless steels from corrosive cargoes such as phosphoric acid is of a low intensity and can therefore be disregarded.
Pitting corrosion might cause severe damage. To avoid this happening it is important to choose the correct type of stainless steel. Generally speaking high molybdenium contents is beneficial in this respect. Pittings may take the shape of small holes, 1-2 mm in depth and diameter to begin with. At times they are almost hidden below the surface of the steel with an "entrance hole" of only a few tenths of a mm. with a cavity of 1-2 mm below. They are therefore sometimes very difficult to detect. So called dye-penetrant tests are useful for detection when one knows approximately where to look for defects. Pitting may develop in a generally corrosive surrounding, especially when the surface is disturbed or when some extra chemical aggressive age.nt is present such as:
i) chlorides (seawater, "salt"). Contents above some 100-200 ppm are generally dangerous when together with some other corrosive agent such as phosphoric acid. Fluorides have a similar effect.
ii) particles of iron-or other materials on the surface
iii) craters or pores in weld deposits
iv) weld slag or slag from rolling mill
v) surface defects, micro cracks, rough surfaces vi) lack of oxygen renders formation of chromium oxide difficult
vii) high temperatures (above ca 400C corrosion rates in crease rapidly)
Crevice corrosion may occur in narrow spaces where the corrosive agent can enter but without circulation, with a lack of oxygen as consequence.
Typical locations:
a) under bolted connections
b) under cargo sediments
c) under paint on a stainless steel surface.
Maintenance of stainless steel
The following rules for maintenance of stainless steel apply:
1. Keep chlorides away. Avoid seawater in the tanks and rinse with freshwater carefully after seawater washing. Seawater must never be permitted to dry up and leave salt crusts on the surface. Keep the hatch covers closed and the airpipes protected to prevent seawater or a saline atmosphere entering the tanks. Preferably change footwear before entering a tank when at sea.
2. Remove any particles or sediments such as rust, particles from grinding operations, cargo sediments (phosphoric acid), "scale". Cargo remains to be removed as soon as possible after discharge.
3. Surface finish. Tank surfaces to be kept bright and free from scratches. This means-that possible corrosion or other mechanical defects should be ground and polished to the original finish. Normally grinding disc "grain 80" can be used, followed by a final operation with "grain 120". Local pittings of substantial depth can be welded, minor pittings ground away.
4. Inspect for corrosion after each cargo, especially tank bottoms and under deck. In order to avoid salt crystals forming on the tank bottom 10-20 cm fresh water is sometimes kept in the tanks on the ballast voyage.
5. Cleaning can normally be carried out with all common cleaning agent's such as "emulsifiers", "solvent cleaners" and alkaline cleaners as well as caustic soda.
6. Stainless steel tanks are sometimes passivated by application of 12- 15 % nitric acid (HNO 3) . This acid is strongly oxidizing. The procedure assists in building up the passive chromium layer on the steel, thus increasing its chemical resistance. Passivation is normally carried out after tank surface repairs in order to assist the normal passivation in air. If aggressive cargoes are to be loaded within 24 hours all repairs must be passivated. In practice "passivation" with nitric acid is often used for the removal of discolorations and particle contaminations on the surface. In factlit is being more used as a thorough cleaning agent than a passivating chemical. Passivation is normally carried out with a brush or, for a whole tank, by spraying, After 10-20 min the acid should be washed off with large amounts of fresh water.
IMPORTANT: Nitric acid gives off nitrous gases which are very toxic (with delayed effects). Ventilate completely and use breathing masks for larger areas in confined spaces! The atmosphere can be tested for nitrous gases by means of test tubes . Use protective clothing and goggles. Nitric acid is usually available in 60 1/o concentration. Be careful: spills may cause self-ignition of organic matter.
7. Pickling is the toughest way of cleaning stainless steel. This method is used for the removal of welding slag, oxides and discolorations from welding or discoloration from cargoes. Pickling paste, consisting of, among other components, nitric acid and hydrofluoric acid shall be applied with the same precautions as nitric acid above. Pickling should be followed by passivation with nitric acid. Pickling involves a lot of work and can only be used on relatively small areas, unless carried out by specialists.
Fig : Steel composition
Other Info pages
Cuprous alloys for shipbuilding of seagoing chemical tankers
Copper and its alloys corrode in many cargoes and may contaminate them, e g styrene, phenol, vinyl chloride, aniline, ammonia solutions etc. Check your cargo against information . Particularly aggressive are the ammonia compounds; they cause inter -crystalline corrosion of cuprous alloys very rapidly. The object in question disintergrates very soon. ....
Use of magnesium and alluminium alloys as sacrificial anodes
Alloys of magnesium and aluminium should never be used in the cargo tank area, due to their poor corrosion resistance in such environments. .....
Introducing rubber lined tanks for the transport of phosphoric acid, waste acids and hydrochloric acid
In recent years a number of ships have been fitted with rubber lined tanks, for the transport of phosphoric acid, waste acids and hydrochloric acid. .....
Tools for shipboard maintenance
Although grit blasting and the use of mechanically powered tools are not normally considered to fall within the definition of hot work, both these operations should only be permitted under controlled conditions. ......
Sea transport of various liquid chemicals at sea
Chemical tankers primarily transport organic and inorganic chemicals as well as vegetable oils and fats. The total global volume of chemicals is estimated at approximately 60 million metric tonnes per year. In addition, the transportation of vegetable oils, alcohols, molasses and lubricating oils amounts to 40 - 45 million tonnes per year. .....
stainless steel as shipbuilding materials for chemical tankers
Iron, steel Mild steel and high tensile steel is, and will continue to be the most important material in the building of chemical tankers and their cargo tanks.Steel is attacked by only a few products, mainly acids and, of course ballast and washing water. Steel itself contaminates very few products, one of them being high purity caustic soda. .... ....
Types of various chemical tankers at sea
modem chemical tanker is primarily designed to carry some of the several hundred hazardous products now covered by the IMO Bulk Chemical Codes. The following general types of chemical carriers have developed since the trade began: ....
Main Info pages!
Home page ||| Chemical hazards ||| Cargo planning & Stowage ||| Cargo loading ||| Cargo documents ||| Safe stability ||| Cargo care ||| Preparation for unloading ||| Inert gas systems |||Gas freeing ||| Nitrogen handling ||| Chemical handling Safe practice |||Handling equipments ||| Cargo & Ballast pumps ||| Cargo tanks |||Tank cleaning |||Special cargoes |||Spills emergencies |||Fire protection
Chemicaltankerguide.com is merely an informational site about various aspects of chemical tankers and safety tips that may be particular value to those working in: Chemical Handling, Chemical Storage, Liquefied Chemical Suppliers, Chemical Shipping, Chemical Transportation, Chemical Terminals, Bulk Chemical Services and Chemical Processing. If you are interested in finding out more about chemical tanker guideline please visit IMO official website. For any comment please Contact us
Copyright © 2011 Chemical Tanker Guide.com All rights reserved.
